how many electrons in cu|Copper : Tagatay In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple. When we write the configuration we'll put all 29 electrons in orbitals . STL RESULT TODAY – The Philippine Charity Sweepstakes Office (PCSO) Releases the STL lotto result today for September 03, 2024, in Luzon, Visayas, and Mindanao at 10:30 AM, 3 PM, 7 PM, and 8 PM. PCSO departmentalizes Small Town Lottery (STL) draws, held daily from Monday to Sunday. STL Result Today September 03, .
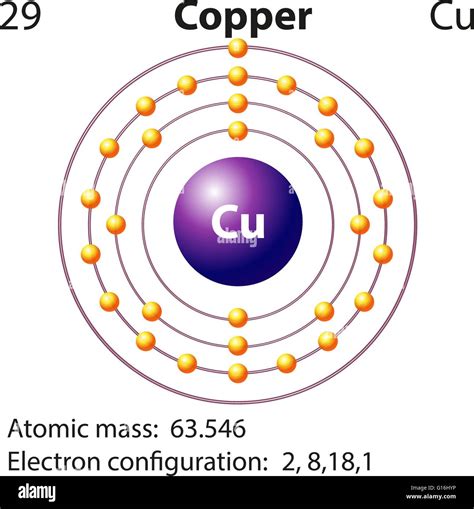
how many electrons in cu,In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple. When we write the configuration we'll put all 29 electrons in orbitals .

When we write the configuration we'll put all 20 electrons in orbitals around the .Since 1s can only hold two electrons the next 2 electrons for magnesium go in .Since 1s can only hold two electrons the next 2 electrons for Potassium go in the .When we write the configuration we'll put all 14 electrons in orbitals around the .
The next six electrons will go in the 2p orbital. The p orbital can hold up to six .
Lithium is the third element with a total of 3 electrons. In writing the electron .Copper (Cu, Cu +, Cu 2+) Iron (Fe, Fe 2+, Fe 3+) Read my article in Science .Fluorine is the ninth element with a total of 9 electrons. In writing the electron . Cu – e – → Cu + Here, the electron configuration of copper ion(Cu +) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This copper ion(Cu +) has twenty-nine protons, thirty-five neutrons, and twenty-eight electrons. . In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Copper (Cu). From the Periodic Table .
Copper is the 29th element in the periodic table and has a symbol of Cu and atomic number of 29. It has an atomic weight of 63.546 and a mass number of 63. Copper has twenty .
Element Copper (Cu), Group 11, Atomic Number 29, d-block, Mass 63.546. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main .Atomic Number: 29. Atomic Mass: 63.546 amu. Melting Point: 1083.0 °C (1356.15 K, 1981.4 °F) Boiling Point: 2567.0 °C (2840.15 K, 4652.6 °F) Number of Protons/Electrons: 29. .

The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Copper is 29. Each electron is influenced by the electric .Key Takeaways. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the stability gained from a half-filled or fully-filled . The atomic number of copper is 29. It is a ductile, malleable, and soft metal that has a very high electrical and thermal conductivity. Copper Electron Configuration. The surface of pure copper that is .8 protons and 10 electrons b. 16 protons and 8 electrons c. 8 protons and 8 electrons d. 10 protons and 8 electrons e. none of the above How many electrons in a S atom has n = 2? a.Valence Electrons: The number of electrons present in the outermost or last shell of an atom is known as its valence electrons. The number of valence electrons present in a given molecule, mostly also represents its group number. Valence electrons of Copper : The ground state electronic configuration of Cu is given as: 1 s 2 2 s 2 2 p 6 3 s 2 3 .
You can see that all the d-orbitals are completely filled (all the 10 electrons are present) in the elemental state (Cu) but they are incomplete (only 9 electrons are present) in their oxidation state (Cu .Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 63 Cu Electron configuration [Ar] 3d 1 0 4s 1 CAS number: 7440-50-8 ChemSpider ID:
Here, the d-orbital is filled with electrons and there is an electron at the last energy shell. Therefore, the valence electrons of copper are one. How many valence electrons does copper ion(Cu +, Cu 2+) have? The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation.The number of unpaired electrons in Cu +atomic number =29 is:A. 0B. 3C. 1D. 2
how many electrons in cu Hint The number of electrons present in the last shell of an atom is known as valence electrons. In order to solve this question, we must remember that the d electrons is used in atomic configuration to describe the electron configuration of the valence electrons of an element. Complete step by step answer: - The atomic number .Copper To find the number of valence electrons for Copper (Cu)we need to look at its electron configuration. This is necessary because Cu is a transition metal (d . Neutral Conditions. The first step to balance any redox reaction is to separate the reaction into half-reactions. The substance being reduced will have electrons as reactants, and the oxidized substance will have electrons as products.
The atomic number of C u is 29 and it forms C u + ion by the loss of one electron. C u 2 + ( Z = 28 ) : [ A r ] 3 d 10 4 s 0 . Hence, C u + ion has no unpaired electron.
For example, how many neutrons does the 65 Cu isotope have? The first thing, again, is to look up the number of protons for Cu, and this is the atomic number. From the periodic table, we find that it has 29 protons. .Because two electrons are required to reduce a single Cu 2 + ion, the total number of moles of Cu produced is half the number of moles of electrons transferred, or 1.2 × 10 −3 mol. This corresponds to 76 mg of Cu. In commercial electrorefining processes, much higher currents (greater than or equal to 50,000 A) are used, corresponding to .Atomic Number – Protons, Electrons and Neutrons in Copper. Copper is a chemical element with atomic number 29 which means there are 29 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e .
To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of . This equation tells us how many electron states are available in a three-dimensional metallic solid. However, it does not tell us how likely these states will be filled. . {28}\) conduction electrons per cubic meter. (a) Calculate its Fermi energy. (b) Compare this energy to the thermal energy \(k_BT\) of the electrons at a room temperature . Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.The chemical symbol for Boron is B. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. There are over 100 different borate minerals, but the most common are: borax, . The layers of electrons around an atom don't always follow a straightforward pattern, so you can't always predict an element's behavior just by counting these outer electrons. Nevertheless, it's useful to know the most common valences. Here is a table of element valences. Table of Element Valences. Number: Element: Valence: 1:how many electrons in cu Copper If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The only difference is at the end of the configuration that is in the 3d and 4s shells. The atoms of copper have enough electrons which completely fill the 3d sub-shell. How Many Valence Electrons Does .
how many electrons in cu|Copper
PH0 · How to find the Number of Protons, Electrons, Neutrons for
PH1 · How Many Protons, Neutrons and Electrons Does
PH2 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH3 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH4 · Electron Configuration For Copper
PH5 · Copper Electron Configuration (Cu) with Orbital Diagram
PH6 · Copper (Cu)
PH7 · Copper
PH8 · Chemical Elements.com